
Insulating correct signaling proteins from inactivation These abilities may be related to stability of the interaction between the scaffold and the kinases, the basal phosphatase activity in the cell, scaffold location, and expression levels of the signaling components. In three-kinase signaling cascades, scaffolds bind all three kinases, enhancing kinase specificity and restricting signal amplification by limiting kinase phosphorylation to only one downstream target. Many hypotheses about how scaffolds coordinate positive and negative feedback come from engineered scaffolds and mathematical modeling. This localization is able to locally regulate PKA and results in the local phosphorylation by PKA of its substrates.Ĭoordinating positive and negative feedback A particular example of this process is the scaffold, A-kinase anchor proteins (AKAPs), which target cyclic AMP-dependent protein kinase ( PKA) to various sites in the cell.
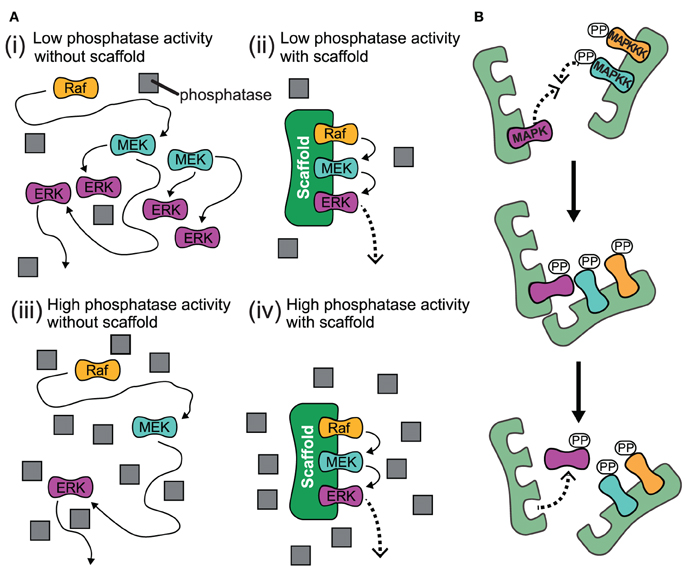
Scaffolds localize the signaling reaction to a specific area in the cell, a process that could be important for the local production of signaling intermediates. Localization of signaling components in the cell Ste5 has been proposed to direct mating signaling through the Fus3 MAPK by catalytically unlocking this particular kinase for activation by its MAPKK Ste7. An example is the Ste5 scaffold in the mitogen-activated protein kinase ( MAPK) pathway. Such changes may be able to enhance or inhibit the activation of these signaling proteins. Scaffolds may also be catalytic as interaction with signaling proteins may result in allosteric changes of these signaling components. Additionally, some signaling proteins require multiple interactions for activation and scaffold tethering may be able to convert these interactions into one interaction that results in multiple modifications. A common example of how scaffolds enhance specificity is a scaffold that binds a protein kinase and its substrate, thereby ensuring specific kinase phosphorylation. This assembly may be able to enhance signaling specificity by preventing unnecessary interactions between signaling proteins, and enhance signaling efficiency by increasing the proximity and effective concentration of components in the scaffold complex.

Scaffolds assemble signaling components of a cascade into complexes. This particular function is considered a scaffold's most basic function. Scaffold proteins act in at least four ways: tethering signaling components, localizing these components to specific areas of the cell, regulating signal transduction by coordinating positive and negative feedback signals, and insulating correct signaling proteins from competing proteins.

Three distinct domains of Ste5 were shown to associate with the protein kinases Ste11, Ste7, and Fus3 to form a multikinase complex. The first signaling scaffold protein discovered was the Ste5 protein from the yeast Saccharomyces cerevisiae. In such pathways, they regulate signal transduction and help localize pathway components (organized in complexes) to specific areas of the cell such as the plasma membrane, the cytoplasm, the nucleus, the Golgi, endosomes, and the mitochondria. Although scaffolds are not strictly defined in function, they are known to interact and/or bind with multiple members of a signalling pathway, tethering them into complexes. In biology, scaffold proteins are crucial regulators of many key signalling pathways.


 0 kommentar(er)
0 kommentar(er)
